
Health Insurance
(Pharmaceutical Benefit) (General Provisions) (No. 2) (Jersey) Order 2002
Official Consolidated
Version
This is an official
version of consolidated legislation compiled and issued under the authority of
the Legislation (Jersey) Law 2021.
Showing the law
from 3 July 2025 to Current

Health Insurance
(Pharmaceutical Benefit) (General Provisions) (No. 2) (Jersey) Order 2002[1]
1 Interpretation
(1) In
this Order, unless the context otherwise requires –
“approved prescribing practitioner” means
any medical practitioner, dentist, optician or other prescribing practitioner
approved by the Minister under Article 26 of the Law;
“Drug Tariff” means the statement compiled
and published by the Secretary of State for Health of the United Kingdom pursuant
to Regulation 18(1) of the National Health Service (Pharmaceutical
Services) Regulations 1992 of the United Kingdom, as that statement is for the
time being in force;
“Health Card” means an Identity Card
issued in accordance with the Health Insurance
(Evidence) (Jersey) Order 2008;
“Law” means the Health Insurance
(Jersey) Law 1967;
“prescription” has the same meaning as given
to the expression “prescribed form” by Article 5.[2]
(2) [3]
2 Approval
of suppliers
(1) An
application by a person conducting a retail pharmacy business for approval
under Article 26(2) of the Law as a supplier of pharmaceutical benefit
must be substantially in the form set out in Part 1 of Schedule 1.
(2) An
application by any other person for that approval must be substantially in the
form set out in Part 2 of Schedule 1.
3 Terms
and conditions of supply of pharmaceutical benefit
For the purpose of Article 26(2)
and (3) of the Law the terms and conditions a person in an application for
approval as an approved supplier must undertake to supply pharmaceutical
benefit on are those set out in Part 1 of Schedule 2.
4 Schemes
for securing proper pharmaceutical services
(1) The
Minister may act in accordance with paragraph (2) if at any time he or she
is satisfied that there is an insufficient number of places of business of
approved suppliers open outside normal business hours.
(2) The
Minister may after consulting the Pharmaceutical Benefit Advisory Committee
prepare a scheme designed to ensure that a sufficient number of places of
business of approved suppliers are open at reasonable times outside normal
business hours.
(3) The
scheme must –
(a) specify
the days and hours during which places of business of approved suppliers are to
be open; and
(b) provide
for payments to be made to those suppliers in respect of periods during which
their premises are open under the scheme.
5 Prescribed
form for the supply of pharmaceutical benefit
(1) For
the purposes of Article 15(2) of the Law “prescribed form”
means a form substantially in the form set out in Schedule 3
that –
(a) contains
the particulars required to complete the form;
(b) is
dated with the date on which it is to become effective;
(c) is
signed by the approved prescribing practitioner that gave the form; and
(d) is
printed, or is created in electronic form to be transmitted as an electronic
communication, as may be specified by the Minister. [4]
(2) A
form is not in the prescribed form if it authorizes the supply of
pharmaceutical benefit for more than one person.
(3) In
paragraph (1)(d), “electronic communication” has the meaning
given in Article 1(1) of the Electronic
Communications (Jersey) Law 2000.[5]
6 Period
of supply of pharmaceutical benefit[6]
(1) An approved prescribing
practitioner, other than a dentist, must not give a prescription for the supply
of pharmaceutical benefit to a person for a period exceeding 90 days.
(2) An approved prescribing
practitioner who is a dentist must not give a prescription for the supply of
pharmaceutical benefit to a person for a period exceeding 30 days.
(3) An approved prescribing
practitioner must not give more than one prescription for the same
pharmaceutical benefit at any one time.
(4) However, an approved
prescribing practitioner may give up to 4 prescriptions in respect of the
same pharmaceutical benefit at any one time if the prescriptions are for
consecutive periods of supply.
(5) An approved prescribing
practitioner must not during a period of treatment give a further prescription
to a person for the same pharmaceutical benefit unless the practitioner is
satisfied that it is necessary or desirable to do so for the purpose of the
treatment and –
(a) the
pharmaceutical benefit is intended for the continuation of the treatment after
the expiry of the present period of treatment and the prescription is dated no
earlier than 21 days before the end of that period;
(b) the
prescription is for an increase in dosage; or
(c) the
prescription is to replace pharmaceutical benefit previously supplied on
prescription and destroyed or lost.
(6) An approved supplier
must not supply pharmaceutical benefit ordered on a prescription –
(a) before
the effective date of the prescription; or
(b) which
does not comply with the requirements of this Article.
7 Supply
(1) This
Article applies where in accordance with a prescription pharmaceutical benefit
is supplied by an approved supplier in respect of an insured person or a
dependant of an insured person.
(2) The
person to whom the pharmaceutical benefit is supplied must produce and show to
the approved supplier the insured person’s Health Card.
(3) Subject
to paragraph (4) –
(a) the person
to whom the pharmaceutical benefit is supplied must surrender the prescription
to the approved supplier; and
(b) the
approved supplier must mark on the prescription the health insurance number specified
on the insured person’s Health Card.
(4) The
person taking delivery of pharmaceutical benefit is not required to surrender
the prescription if, by electronic means, the approved prescribing practitioner
who signed the prescription –
(a) notifies
the approved supplier of the particulars contained in the prescription and its
effective date;
(b) confirms
that the prescription is signed by the practitioner; and
(c) undertakes
to surrender the prescription to the approved supplier within 72 hours of the notification,
and the approved supplier
is satisfied that, by reason of an emergency, the practitioner has been unable
to furnish the person taking delivery of the pharmaceutical benefit with the
prescription so that it is available for surrender at the time of delivery.[7]
(5) [8]
(6) [9]
(7) [10]
(8) [11]
8 Offences
(1) Except
as provided by paragraph (2), a person who fails to comply with a
provision of this Order shall be guilty of an offence and liable to a fine not
exceeding level 2 on the standard scale.
(2) A
person who fails to surrender a prescription as required under Article 7(3)(a)
is liable to a fine not exceeding level 1 on the standard scale.
9 Calculation
of prescription costs payable by the Minister[12]
(1) The amount to be paid
by the Minister to an approved supplier for each item of pharmaceutical benefit
supplied on a prescription in respect of an insured person or a dependant of an
insured person is the sum of –
(a) the
basic price of the ingredients calculated in accordance with the Drug Tariff as
at the date of supply;
(b) the
appropriate basic dispensing fee in Table 1 of Schedule 5; and
(c) any
additional dispensing fee or fees in Table 2 of Schedule 5.
(2) However, if
paragraph (3) applies, the amount to be paid under paragraph (1) is
the amount that would be payable under that paragraph if each instalment had
been supplied on a separate prescription.
(3) This paragraph applies
if the prescription –
(a) was
signed by an approved medical practitioner;
(b) specifies
that it is to be supplied in instalments at stipulated intervals or on given
dates; and
(c) at
the time of supply is for a substance or product that is in Part 1, 2 or 3
of Schedule 2 to the Misuse of Drugs (Jersey) Law 1978.
10 Citation
This Order may be cited
as the Health Insurance (Pharmaceutical Benefit) (General Provisions)
(No. 2) (Jersey) Order 2002.
Schedule 1
(Article 2)
APPROVAL OF SUPPLIERS OF PHARMACEUTICAL BENEFIT
PART 1
HEALTH
INSURANCE (JERSEY) LAW 1967
Form of application
for approval as supplier of pharmaceutical benefit of person(s) lawfully
conducting a retail pharmacy business
|
To: The Minister for Social Security
I/We, ....................................................................................................
of .........................................................................................................
being a person or persons lawfully conducting a retail pharmacy
business within the meaning of the Medicines (Jersey)
Law 1995, apply for approval in accordance with Article 26(2)
of the Health
Insurance (Jersey) Law 1967. I/we undertake to dispense medicines
and supply drugs at the prices fixed and in accordance with the terms and
conditions prescribed under the Health Insurance
(Jersey) Law 1967. I/We understand that those prices, terms and
conditions are subject to variation in the manner provided by that Law.
The address(es) of my/our business premises registered in
accordance with the Medicines
(Jersey) Law 1995 and the pharmacist(s) in charge of those premises
will be as follows –
|
|
Address(es)
of premises
|
Full
name(s) of pharmacist(s) in charge
|
|
|
|
|
Signed:..................................................................................................
Date:
....................................................................................................
|
PART 2
HEALTH
INSURANCE (JERSEY) LAW 1967
Form of application
for approval as supplier of pharmaceutical benefit of person(s) other than
person(s) lawfully conducting a retail pharmacy business
|
To: The Minister for Social Security
I/We ......................................................................................................
of ..........................................................................................................
apply for approval in accordance with Article 26(3) of the Health Insurance
(Jersey) Law 1967. I/we undertake to supply drugs (except poisons in
Part 1 of the Poisons List set out in the Schedule to the Poisons List (Jersey)
Order 1986) at the prices fixed and in accordance with the terms and
conditions prescribed under the Health Insurance
(Jersey) Law 1967. I/We understand that those prices, terms and conditions
are subject to variation in the manner provided by that Law.
The address(es) of my/our business premises for this purpose will be
........
.............................................................................................................
Signed: ..................................................................................................
Date: ......................................................................................................
|
Schedule 2[13]
(Article 3)
PART 1
TERMS AND CONDITIONS TO BE OBSERVED BY AN APPROVED SUPPLIER
1 Supplier
to supply pharmaceutical benefit
(1) The
supplier must supply pharmaceutical benefit with reasonable promptness to a person
who presents a prescription for them.
(2) Sub-paragraph (1)
does not require a supplier to supply a pharmaceutical benefit that the
supplier does not ordinarily supply.
(3) If
under this paragraph a supplier is required to supply a medicine or drug the
supplier must supply the medicine or drug in a suitable container
being –
(a) in
relation to capsules, tablets, pills or any other medicine or drug in solid
form (other than those prepacked in foil or paper-board or strip card
containers by the manufacturer) – an airtight container of glass,
aluminium or rigid plastic;
(b) in
relation to ointments, creams or pastes (other than those prepacked by the
manufacturer) – a container of glass, aluminium or rigid plastic;
(c) in
relation to eye, ear or nasal drops (other than those prepacked by the
manufacturer) – a container of glass either incorporating or having a
separate dropper attachment;
(d) in
relation to liquid medicines (other than those prepacked by the manufacturer)
– a container of glass or rigid plastic, including, in the case of an
oral liquid medicine, a 5 ml. plastic measuring spoon (unless the patient
already has one or the manufacturer’s pack includes one).
(4) The
supplier must not give, promise or offer a gift or a reward as an inducement to
or in consideration of a person presenting a prescription to the supplier.
2 Place
and hours of business
(1) The
supplier must supply pharmaceutical benefit at the place or places of business
specified in the supplier’s application for approval under Article 26
of the Law.
(2) The
supplier must keep that place or those places open for the supply of
pharmaceutical benefit –
(a) during
normal business hours; and
(b) on
the days and during the hours specified in any scheme made by the Minister
under Article 4.
(3) At
each such place of business the supplier must display a notice to be provided
by the Minister in the form set out in Part 2 or Part 3 of this Schedule.
(4) If
the supplier is a person lawfully conducting a retail pharmacy business the
supplier must also display a notice to be provided by the Minister in the form
set out in Part 4 of this Schedule when the supplier’s place of
business is closed.
(5) The
notice must indicate –
(a) the
addresses of other people lawfully conducting a retail pharmacy business where
medicines and drugs may be obtained; and
(b) the
times when they may be obtained at those premises.
(6) Each
notice must be displayed in a manner that makes it easily visible to members of
the public.
3 Dispensing
medicines
The supplier must ensure that
the supply of medicines on prescriptions is performed by or under the
supervision of a pharmacist.
4 Names
of pharmaceutical chemists
Whenever required to do
so by the Minister the supplier must furnish to the Minister the name of each
pharmacist employed by the supplier in dispensing medicines on prescription.
5 Charges
Except for charges that
are required or authorized to be made by this or any other Order made under the
Law the supplier must supply a pharmaceutical benefit and any container free of
charge.
6 Advertising
(1) The
supplier must not advertise either directly or by implication that the supplier
is an approved supplier or that the supplier provides or is authorized to
provide pharmaceutical benefit.
(2) Despite
sub-paragraph (1) the supplier may –
(a) display
a notice required by paragraph 2;
(b) include
in an advertisement a statement of the days and hours at which pharmaceutical
benefit is supplied.
7 Information to be provided
(1) This
paragraph does not apply except where the Minister requires information to
determine the amount payable under Article 10 to the supplier for
pharmaceutical benefit supplied by the supplier.
(2) The
supplier must furnish to the Minister or to such person or body as the Minister
directs information that the Minister requires concerning so much of the
supplier’s business that relates to the supply of pharmaceutical benefit.
(3) The
supplier must permit a person authorized in writing to do so by the Minister to
conduct surveys at each place of business at or from which the supplier
supplies pharmaceutical benefit.
8 Payment
(1) On
dates specified by the Minister the supplier must furnish to the Minister or to
such person or body as the Minister directs the prescriptions on which
pharmaceutical benefit has been supplied by the supplier.
(2) The
prescriptions must be arranged in the manner the Minister directs and must be
accompanied by a declaration.
(3) The
declaration must contain such particulars relating to the supply by the
supplier of pharmaceutical benefit as the Minister specifies.
9 Withdrawal
If the supplier wishes to
cease to be an approved supplier the supplier must give at least 3 months
written notice to the Minister (or such shorter notice as the Minister may
agree) that the supplier no longer wishes to supply pharmaceutical benefit.
PART 2
Form of notice to be displayed by an
approved supplier who is a person lawfully conducting a retail pharmacy
business
Health Insurance Scheme
(Name of approved
supplier)
Approved under the Health Insurance
(Jersey) Law 1967 to dispense medicines and supply drugs.
These premises are open
at the following times –
PART 3
Form of notice to be displayed by an
approved supplier other than a person lawfully conducting a retail pharmacy
business
Health Insurance Scheme
(Name of approved
supplier)
Approved under the Health Insurance
(Jersey) Law 1967 to supply drugs (except poisons in Part 1 of
the Poisons List set out in the Schedule to the Poisons List (Jersey)
Order 1986).
These premises are open
at the following times –
PART 4
Form of notice to be displayed by an
approved supplier who is a person lawfully conducting a retail pharmacy
business at times when the person’s premises are closed
Health Insurance Scheme
When these premises are
closed, medicines and drugs may be obtained at the addresses and times shown
below –
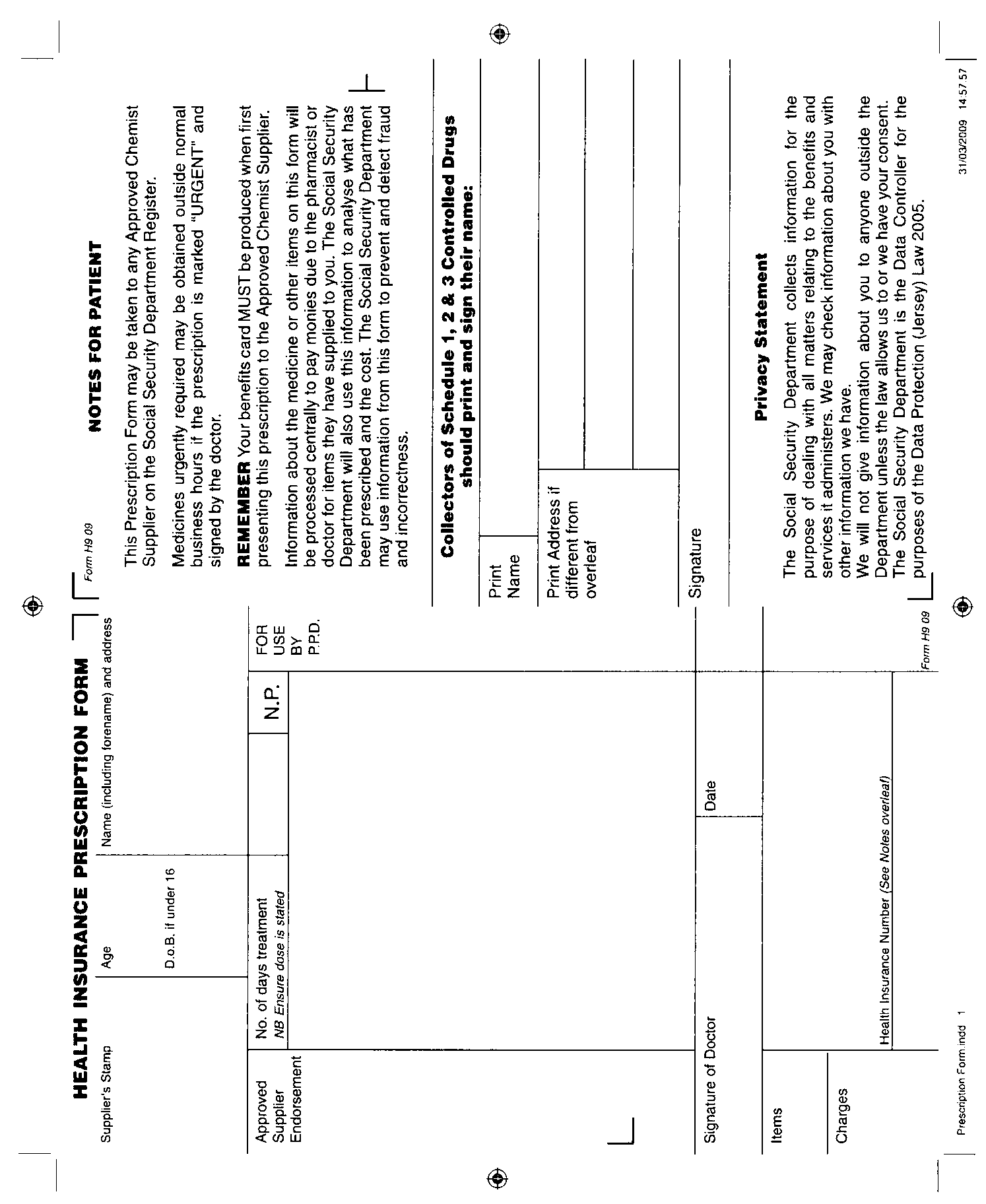
Schedule 3[14]
(Article 5)
Form of prescription
Schedule 4[15]
Schedule 5[16]
(Article 9(1)(b) and
(c))
dispensing
fees
TABLE 1:
BASIC DISPENSING FEES
|
Date of supply and
description
|
Fee in pence for each item
of pharmaceutical benefit supplied on a prescription
|
|
In the period of 8 months
beginning on 1st May 2023 –
|
|
|
for supply by an approved
supplier of each of the first 50,000 items of pharmaceutical benefit
|
401
|
|
for supply by an approved supplier
of each item of pharmaceutical benefit after the first 50,000 items
|
340
|
|
In 2024 –
|
|
|
for supply by an approved
supplier of each of the first 50,000 items of pharmaceutical benefit
|
433
|
|
for supply by an approved
supplier of each item of pharmaceutical benefit after the first 50,000
items
|
367
|
|
In 2025 and for each
ensuing period of 12 months –
|
|
|
for supply by an approved
supplier of each of the first 50,000 items of pharmaceutical benefit in
each calendar year
|
467
|
|
for supply by an approved
supplier of each item of pharmaceutical benefit in each calendar year after
the first 50,000 items
|
396
|
TABLE 2:
ADDITIONAL DISPENSING FEES
|
Description
|
Fee in pence for each
item of pharmaceutical benefit supplied on a prescription
|
|
Prescription endorsed
“C.D.” by the approved supplier for drugs listed in
Schedules 2 or 3 to the Misuse of Drugs (General Provisions) (Jersey) Order 2009
|
128
|
|
Prescriptions endorsed
“C.D.” by the approved supplier for chlordiazepoxide, diazepam,
flurazepam, lorazepam, nitrazepam, oxazepam, zolpidem or zopiclone
|
43
|
|
Urgent additional
dispensing fees (see Notes)
|
|
|
Category 1
|
1756
|
|
Category 2
|
2118
|
|
Expensive items additional
dispensing fees
|
|
|
Cost of item of
pharmaceutical benefit –
|
|
|
£75 –
£99.99
|
300
|
|
£100 –
£199.99
|
500
|
|
£200 –
£499.99
|
1000
|
|
£500 or over
|
2500
|
Notes
(1) Category 1
urgent additional dispensing fees are payable only if –
(a) the
prescription is endorsed –
(i) “URGENT”
by the approved prescribing practitioner, and
(ii) by
the approved supplier with the date and time of dispensing;
(b) the
prescription is supplied on a day other than a Sunday or a public holiday; and
(c) the
supply is made –
(i) when the
supplier’s premises are not open for supply, and
(ii) on
the same day the prescription is written, or the following day if supplied
after midnight.
(2) Category 2
urgent additional dispensing fees are payable only if –
(a) the
prescription is endorsed –
(i) “URGENT”
by the approved prescribing practitioner, or “DISPENSED URGENTLY”
by the approved supplier, and
(ii) by
the approved supplier with the date and time of dispensing;
(b) the
prescription is signed by the patient (or the patient’s representative);
(c) the
prescription is supplied on a Sunday or a public holiday; and
(d) the
supply is made –
(i) when the
supplier’s premises are not open for supply, and
(ii) on
the same day the prescription is written, or the following day if supplied
after midnight.
(3) The
urgent additional dispensing fees are not payable for prescriptions supplied by
an approved supplier when the supplier’s premises are open for supply in
accordance with a scheme prepared by the Minister under Article 4.
Schedule 6[17]
Endnotes
Table of Legislation
History
|
Legislation
|
Year and No
|
Commencement
|
|
Health Insurance (Pharmaceutical Benefit) (General
Provisions) (No. 2) (Jersey) Order 2002
|
R&O.48/2002
|
1 October 2002
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment) (Jersey) Order 2003
|
R&O.85/2003
|
4 October 2003
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 2)) (Jersey) Order 2004
|
R&O.90/2004
|
1 October 2004
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 3) (Jersey) Order 2004
|
R&O.99/2004
|
1 October 2004
|
|
States of Jersey (Amendments and Construction
Provisions No. 8) (Jersey) Regulations 2005
|
R&O.48/2005
|
9 December 2005
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 4) (Jersey) Order 2007
|
R&O.185/2007
|
21 January 2008 (applies to benefit supplied
on or after 1 October 2007)
|
|
Health Insurance (Consequential Amendments)
(Jersey) Order 2008
|
R&O.15/2008
|
28 January 2008
|
|
Health Insurance (Pharmaceutical Benefit)
(No. 2) (Amendment No. 5) (Jersey) Order 2008
|
R&O.19/2008
|
1 February 2008
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 6) (Jersey) Order 2008
|
R&O.122/2008
|
1 October 2008
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 7) (Jersey) Order 2009
|
R&O.35/2009
|
1 May 2009
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 8) (Jersey) Order 2009
|
R&O.98/2009
|
1 November 2009
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 9) (Jersey) Order 2010
|
R&O.99/2010
|
1 November 2010
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 10) (Jersey) Order 2013
|
R&O.44/2013
|
1 May 2013
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 11) (Jersey) Order 2013
|
R&O.119/2013
|
1 October 2013
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 12) (Jersey) Order 2014
|
R&O.141/2014
|
1 October 2014
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 13) (Jersey) Order 2014
|
R&O.174/2014
|
1 December 2014
|
|
Health Insurance (Pharmaceutical Benefit)
(General Provisions) (No. 2) (Amendment No. 14) (Jersey) Order 2014
|
R&O.102/2017
|
13 October 2017
|
|
Health Insurance (Approved Prescribing Practitioners
– Midwives and Nurses) (Jersey) Order 2018
|
R&O.55/2018
|
30 April 2018
|
|
Health Insurance (Pharmaceutical Benefit) (General
Provisions) (No. 2) (Amendment No. 15) (Jersey) Order 2023
|
R&O.23/2023
|
1 May 2023
|
|
Health Insurance (Approved Prescribing Practitioners)
(Jersey) Order 2025
|
R&O.34/2025
|
3 July 2025
|
Table of Renumbered
Provisions
|
Original
|
Current
|
|
1(2)(b)
|
spent, omitted from this revised edition
|
|
1(2)(c)
|
1(2)(b)
|
|
10
|
spent, omitted from this revised edition
|
|
11
|
10
|
Table of Endnote References
[1] This
Order has been amended by the States of Jersey (Amendments and Construction
Provisions No. 8) (Jersey) Regulations 2005. The amendments replace all
references to a Committee of the States of Jersey with a reference to a
Minister of the States of Jersey, and remove and add defined terms
appropriately, consequentially upon the move from a committee system of
government to a ministerial system of government
[2] Article 1(1) amended
by R&O.15/2008, R&O.55/2018, editorial change, definition “Health
Card”, “Order 2007” deleted, “Order 2008”
inserted instead
[3] Article 1(2) deleted
by R&O.23/2023
[4] Article 5(1) amended
by R&O.55/2018, R&O.34/2025
[5] Article 5(3) inserted
by R&O.34/2025
[6] Article 6 substituted
by R&O.23/2023
[7] Article 7(4) amended
by R&O.55/2018
[8] Article 7(5) repealed
by R&O.19/2008
[9] Article 7(6) repealed
by R&O.19/2008
[10] Article 7(7) deleted
by R&O.15/2008, repealed by R&O.19/2008
[11] Article 7(8) deleted
by R&O.15/2008, repealed by R&O.19/2008
[12] Article 9 substituted
by R&O.23/2023
[13] Schedule 2 amended
by R&O.19/2008, R&O.23/2023
[14] Schedule 3 substituted
by R&O.35/2009
[15] Schedule 4 substituted
by R&O.102/2017, amended by R&O.55/2018, deleted by R&O.23/2023
[16] Schedule 5 amended
by R&O.85/2003, R&O.90/2004, R&O.185/2007, R&O.122/2008,
R&O.98/2009, R&O.99/2010, R&O.44/2013, R&O.119/2013,
R&O.141/2014, substituted by R&O.23/2023
[17] Schedule 6 substituted
by R&O.141/2014, deleted by R&O.23/2023